Exactech Lawsuit: Recall of Knee, Ankle, and Hip Replacement Devices
Exactech Recall Lawsuit: Knee, Ankle, and Hip Replacement Devices
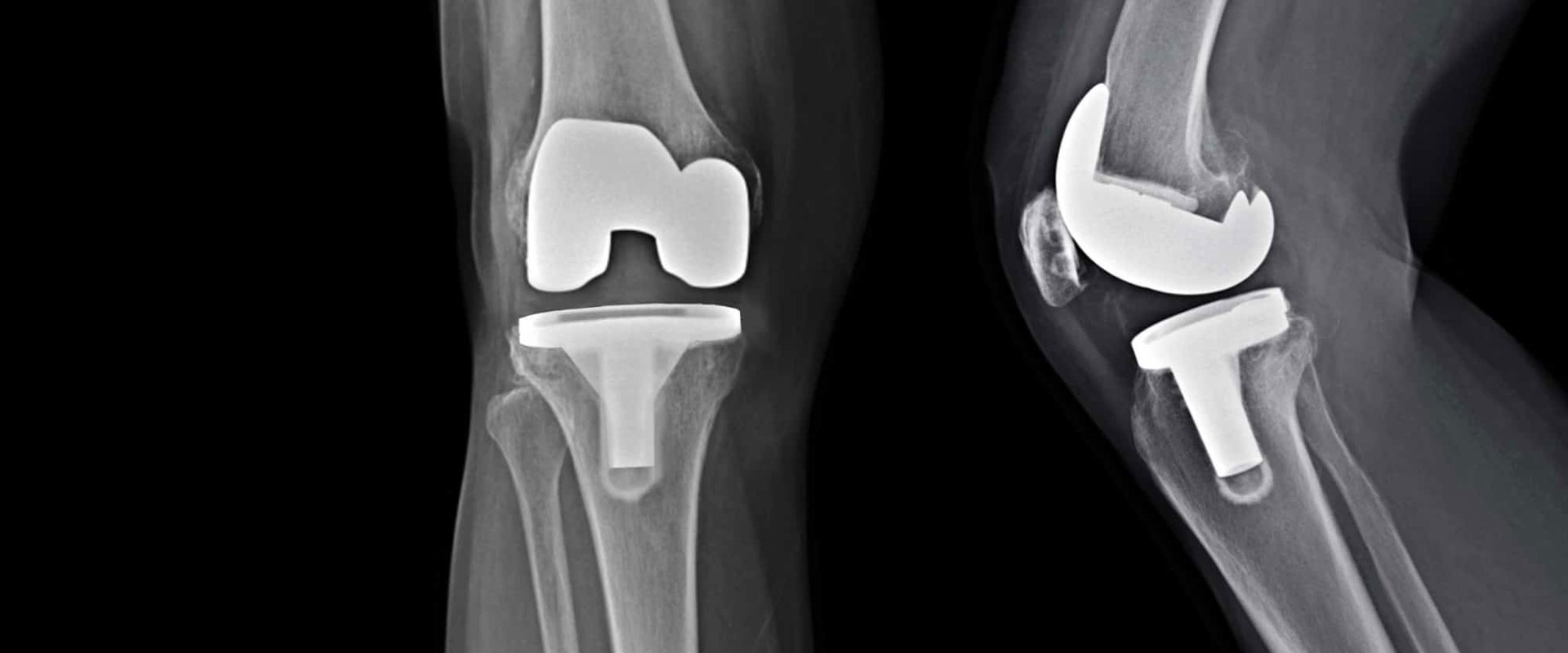
The Exactech lawsuit has brought significant attention to the recall of certain medical devices and their potential risks. In February 2022, Florida-based medical device company Exactech issued a recall on over 150,000 knee, ankle and hip replacement devices. If you or a loved one has received a knee replacement, hip replacement, or ankle replacement since 2004, one of these non-conforming devices may have been used in your procedure. If this is the case, you may be at risk of premature wear on the devices or bone loss. You may even need revision surgery. If you are among the thousands of Americans in this position, you should reach out to an Exactech recall lawyer, as you may be eligible to join an ongoing Exactech lawsuit.
Exactech is a well-established manufacturer of medical technology, and its replacement devices have been used in thousands of procedures across the country. In 2021, however, the company discovered that thousands of parts used in ankle, knee and hip replacement surgeries since 2004 were packaged with a non-conforming vacuum bag, causing a reduction in the shelf-life of the polyethylene constituents. The faulty devices do not contain the technology required to ensure their longevity. They are likely to wear out earlier than they are supposed to, meaning that the patients will need to undergo continued or renewed medical procedures. This will lead to ongoing medical costs, as well as the pain, discomfort and possible lost income that will result from the necessary operations and subsequent recovery time.
$12.5
Million
Spam Text Messages
$11.45
Million
Spam Text Message
$7.00
Million
Spam Text Message






