
DRIVEN BY RESULTS,
COMMITTED TO EXCELLENCE.
Your All-in-One Class Action Attorneys
OVER $1B RECOVERED
FOR CONSUMERS NATIONWIDE.
Shamis & Gentile, P.A. consistently delivers outstanding legal services nationwide. We stand out for our extensive experience, expertise, and resources, allowing us to handle a wide range of cases, including class actions, mass torts, and mass arbitrations.
Our Values
Results-Driven:
No fees until we win.
Integrity:
Transparency, consistent, and cohesive communication every step of the way.
Excellence:
We work tirelessly and quickly until we deliver the results you desire.
Client-First:
As your trusted partners, we are committed to servicing you around the clock, every day.
Class Action Investigations
When you choose us to be your class action lawyers and attorneys, there are no fees or expenses unless we manage to win the case on your behalf.
Data Breach
Bank Overdraft Fee
Spam Text Message
Total Loss Car Accident

Success Stories
Shamis & Gentile, P.A. has Won
Eight Figure
Settlements for our Clients
NATIONWIDE
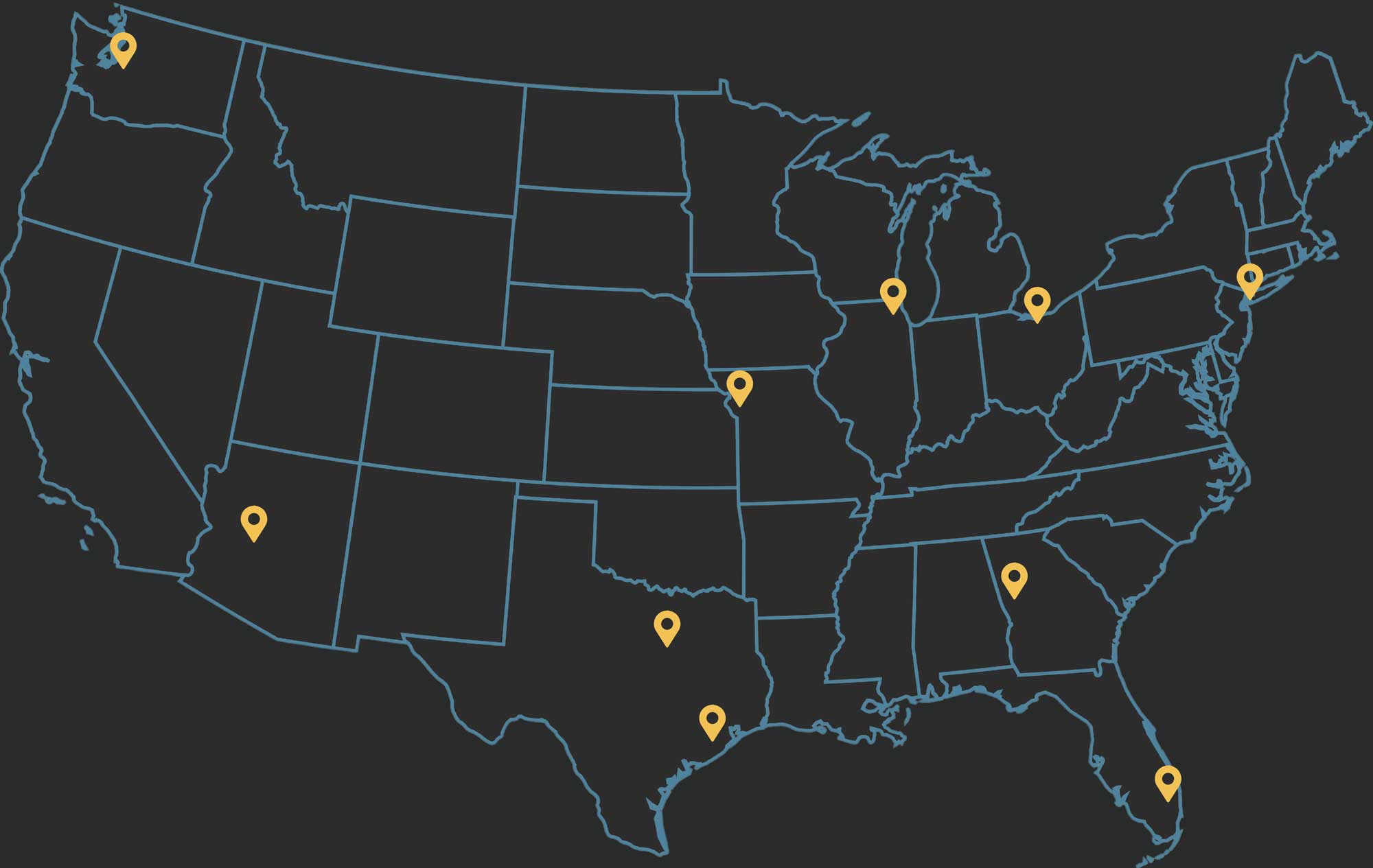
Outstanding legal services to class members across the country. Attorneys licensed in Florida, New York, Texas, Georgia, Ohio, Illinois, Arizona, Missouri, and Washington.
We have recovered over $1billion with over 100,000 cases litigated.
$12.5
Million
Class Action
$2.85
Million
Class Action
$2.85
Million
Class Action
$2.75
Million
Class Action






